What Is Electrolysis of Water
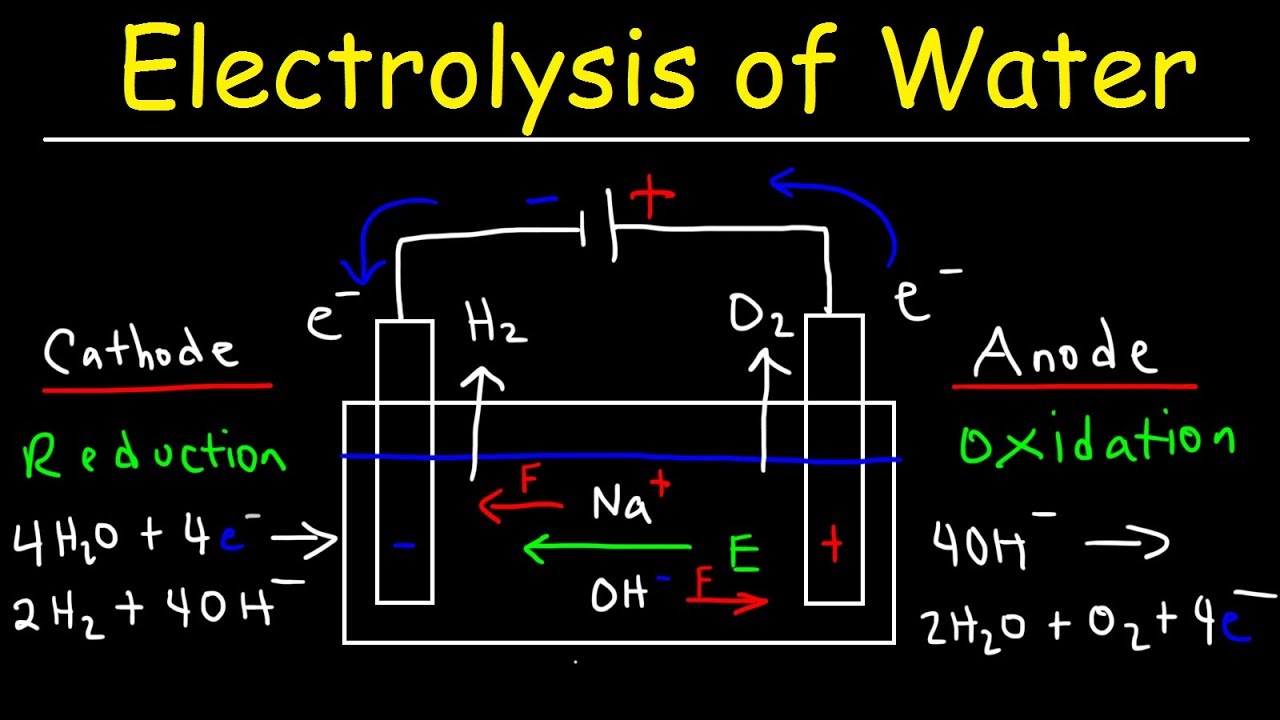
Electrolysis of Water
Electrolysis of water is decomposition of water into oxygen and hydrogen gas due to passage of an electric current. A DC Electri cal power source is connected to two electrodes or two plates. Each electrode attracts ions that are of the opposite charge. Positively chargedions (Cation) move toward the electron providing (Negative) cathode. Negatively charged ions (anion) move toward the electron extracting (positive) anode. In this process electrons are either absorbed or released Neutral atoms gain or loss electrons and become charged ions. That then pass in to the electrolyte. Electro chemically reduced water near by a cathode is hydrogen molecule- rich water. Electrolyzed oxidized water near an anode contains oxygen gas.
